If you wish to understand the German medical cannabis challenges you need to begin with market education, Products (Flowers) and EU-GMP.
The German medical cannabis market is the most attractive one today. After attending the Cannabis Business Conference 2018 in Frankfurt, Germany by Manetch, this statement has turned into a reality, seeing there all the “big boys” of the industry, representing billion dollar companies.
These companies are the ones that are pushing the market forward with big money, big cultivation and processing facilities and huge production capacities. Their stock market projections and market cap are definitely showing to the world the huge market potential of this industry with real numbers, real studies/trials and real people with real stories. Attending were names like Aurora (CA), Canopy Growth (CA), Nuuvera/Aphria (GER/CA), CC Pharma (GER), BOL Pharma (ISR), Seàch (ISR) and many more. All looking at the number one medical cannabis market in the world, the European Union and in its forefront, the potentially biggest market in the world, Germany.
Germany’s Cannabis Business Conference was small, intimate and high quality. There was, as usual, talks about medical cannabis market potential and big money, but unlike other trade shows and expos where they talk about what they are going to do, this conference was addressed by those already doing it. Those companies who already sell, export, import and treat patients.

The reality is much higher than expected: A comparison between Germany’s potential market to the flourishing Canadian current market. (Nuuvera/Aphria)
Clinical trials in autistic children
Dr. Tamir Gido, from BOL Pharma (ISR) gave his speech about his company’s’ clinical trials regarding autistic children. He really moved the people there, even if in a somewhat intensely fashion as he does sometimes. He was met with big applause and a bit unease from the crowd regarding stories of autistic children his company treats. His words brought great understanding of how crucial medical cannabis (THC and CBD alike) is to the health and prosperity of the young and old who until just recently had no solution for their illness.
Understanding The Huge Market Education Challenge
Germany has more than 300k doctors and 82 million residents (by comparison Canada has “only” 36 million). These doctors, all except dentists and veterinarians, are now eligible to prescribe cannabis medication, well, flowers only, at present moment. In this statement lies the whole problem of market education, a problem that we at CBD-Testers wish to solve. In order to understand the market education challenge, we need to remember that it refers to 3 different markets:
1) Doctors who need to prescribe
2) Patients who need a prescription
3) Suppliers/pharmacies/wholesalers who need to supply.
Until 2017, Germany had 14,000 patients. As of today, 2018, there are around 30,000 medical cannabis patients in Germany. They are already receiving their cannabis flower medication from well-established companies from Canada and the Netherlands. That’s a 100% scale-up in just 1 year! The potential growth in Germany in the long-term is over 1 million patients where in Canada it is said to be approximately 450,000 by 2024. But there is a catch, even though these “big boys” have a huge capacity of production and all the money in the world to supply the market, they are still lacking the German precision required to supply its market. It’s just not enough, at least for a highly regulated market as the European Union and Germany in particular.
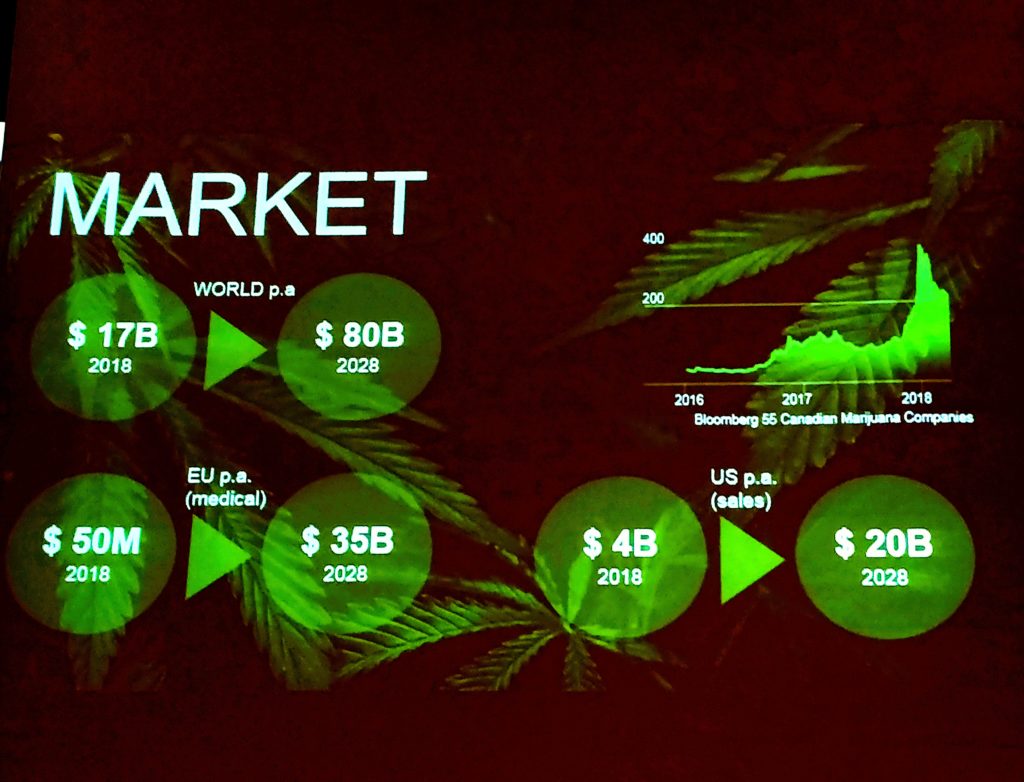
Nuuveras’ insight for the industry 10 years ahead 2018-2028
German Medical Cannabis is only Flowers
When you think about Germany, you think “big pharma”, but cannabis-based drugs is future talk. Cannabis medicine in Germany today is all about cannabis flowers, the strains and the paperwork behind them. Yes, you heard right, flowers. Not patches, pods or pills or anything a German doctor knows and feels comfortable with to prescribe. “Why change what is already working” is a common thought. This imposes a problem.
German Medical Cannabis is EU-GMP compliance
In the next couple of weeks, we are going to go deep and follow-up on German medical cannabis challenges and the regulatory issues of Europe. How it will affect the global medical cannabis industry and its fastly growing side-kick, the CBD hemp industry. From medical grade flowers supplied by “EU-GMP” compliant companies in Canada and Colombia to high CBD hemp flowers and biomass for extractions from Switzerland and the rest of the EU.
One of these regulatory issues is GMP (Good Manufacturing Practice), a word you hear a lot today in the industry. But have you heard about EU-GMP standards? In order to supply Germany a facility needs to be EU-GMP certified, a certification that is assessed by the EMA (European Medicines Agency), the European equivalent to the FDA (Food and Drug Administration). Something that facilities and companies did not take into regard when they built their high-end expensive operations. Sounds difficult, well it certainly is…
German assessors need to asses a non-German company so that supply regardless of the quality and certifications they possess can be delivered to patients in Germany. As of now we wait for the third German tender to be evaluated by the end of November, until then and until the Germans grow their own, Germany is vastly a huge import market. There is big money to be made but in order to do so global companies need to adapt to German regulations.
Keep following us to learn more about German medical cannabis and EU regulations.









